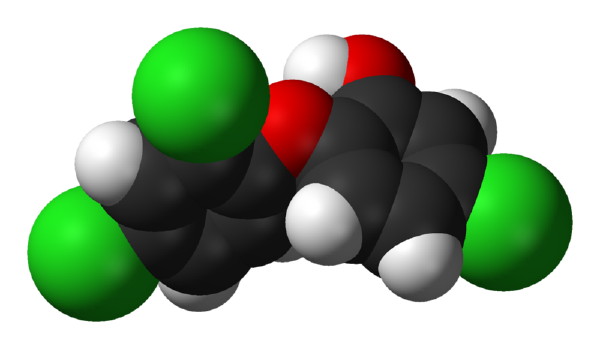
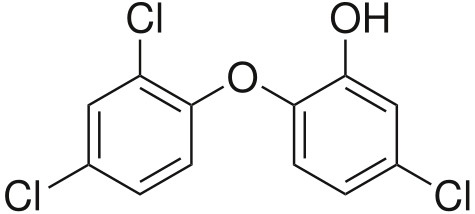
The SCCP, one of the three independent non-food panels of experts that provide the European Commission with scientific advice, considers that “the continued use of triclosan as a preservative at the current concentration limit of maximum 0.3% in all cosmetic products is not safe for the consumer because of the magnitude of the aggregate exposure”.
The SCCP opinion [1] is based on new toxicological data provided by the industry. In particular, data from the percutaneous absorption studies conducted with triclosan [2] indicate that it was relatively well absorbed through the skin in all species tested.
However, “the use of triclosan at a maximum concentration of 0.3% in toothpastes, hand soaps, body soaps/shower gels and deodorant sticks is considered safe,” the SCCP says. Any additional use of triclosan in face powders and blemish concealers at this concentration is also considered safe. Actually, the SCCP considers that well documented studies on human exposure show that that triclosan is safe in these cases.
Nevertheless, the use of triclosan in other leave-on products (e.g. body lotions) and in mouthwashes is not considered safe for the consumer due to the resulting high exposures.
Eventually, the SCCP says that before a final conclusion on the safety of triclosan in cosmetic products can be reached, the potential development of resistance to triclosan and cross-resistance by certain micro-organisms must be assessed. This aspect will be discussed in a separate opinion and is also investigated by the Scientific Committee on Emerging and Newly Identified Health Risks.